Generic Product Development Key steps l Step 5: Formulation and process development
Generic Product Development Key steps
Cont….
A typical product development process can be classified as below
Step 1: Discovery (Product Idea)
Step 2: Product Definition
Step 3: Business Case Development
Step 4: Project feasibility
For Step 1 to step 4, refer link
https://pharmatutor21.blogspot.com/2021/02/generic-product-development-key-steps-l.html
Step 5: Formulation
and process development
Step 6: Pilot
Bioequivalence Study
Step 7: Submission /
Validation Batch Manufacturing
Step 8: Pivotal
Bioequivalence Study
Step 9: Dossier
Filling
Step 10: Post Dossier
Filling
Step 11: Commercial
Launch
Step 12: Post
approval Changes/ Life Cycle Management
Step 5: Formulation and process development
A generic drug is identical, or bioequivalent to a brand name
drug in dosage form, safety, strength, route of administration, quality,
performance characteristics, and intended use. Therefore, understanding of
innovator product is very much essential for the successful development of
Generic product. Literature search and understanding of the content with
respect to the formulation development and linked with the manufacturing
process and bioequivalence study is the prime goal.
Innovator product information:
1.
Identification of Innovator product
1.1 Orange book
information: Follow the below link
https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm
Orange Book:
· · Approved Drug Products with Therapeutic Equivalence Evaluations · List of drug products approved on the basis of safety and effectiveness by the US FDA under the Federal Food, Drug, and Cosmetic Act.
Follow the below steps to Identification of Innovator product
Step-1
Step-2
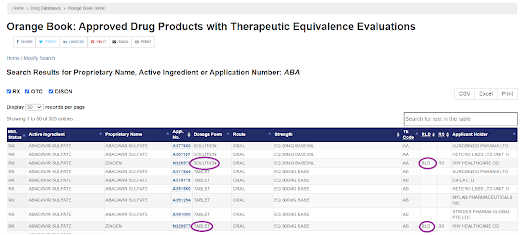
With random search with ‘ABA’ three characters, list stats with Abacavir Sulfate. From the search outcome, it can be learned that
· - Abacavir Sulfate is available as Solution and Tablet dosage form
· - RLD for both these dosage forms are available, same Applicant holder/innovator
· - Generic formulations are available for both Solution and Tablet dosage form
· - Route as Oral
1. How to get the RLD/Innovator product information
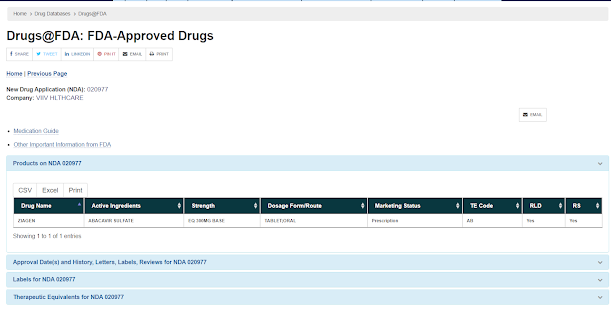
Information can be obtained from: Drugs@FDA: FDA-Approved Drugs
Link: https://www.accessdata.fda.gov/scripts/cder/daf/
Step-1
Lets us enter the ‘Abacavir Sulfate’, as an example
Step-2
Step-3
Step-5
Under Review, below mentioned information are available
Step-6
From this site one can get the information
1. - Labelling: Also known as Patient information leaflet (PIL)
2. - Medical Review
3. - Chemistry of the molecule
4. - Pharmacological information
5. - Microbiological information
6. - Clinical Pharmacology and Bioinformatics information
7. - Administrative information












Noble initiative and Informative. I am sure many of us will find this as a wonderful platform for gaining knowledge and all the best wishes to the blogger.
ReplyDeleteCan we expect a blog on the labelling requirements for Pharmaceuticals?
Hello,
ReplyDeleteIt was a very nicely written article. I like to read it. It was totally informative and topic you covered here, I am totally agreed with you. I must say that I have learnt so many things from it.
Thank you for sharing this article. Keep writing. Thank you so much.
pharmacy app development company
pharmacy app development services